
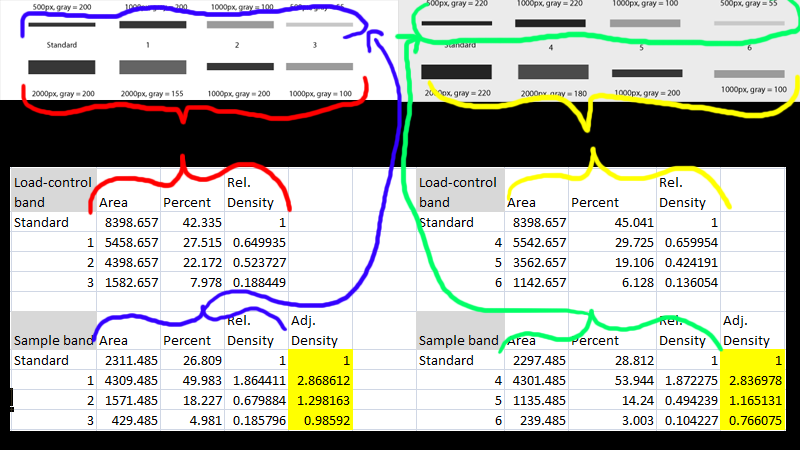
Careful consideration should be given to determine the best approach to prepare samples in order to get a reliable end result. The handling, storage, and lysate preparation of tissue and cell culture specimens can be complicated by the nature of the sample itself and by the large array of reagents and equipment available to prepare them. Validation of the Loaded and Transferred Proteins In this case, the rolling disc for the two lanes analyzed was set to 18 and 25, respectively, such that the peaks of interest were cut at a consistent level between the markers shown with an “X” The rolling disc values were set such that the background was subtracted under the band (i.e., peak) of interest in a uniform manner between the lanes of a given blot.
Background subtraction was set by using the rolling disc setting in the “Lanes” tool. The density of a given band was measured as the total volume under the three-dimensional peak, which could be viewed in two dimensions using the “Lane Profile” tool to adjust the precise width of the band to account for the area under the shaded peak of interest.
#WESTERN BLOT BAND INTENSITY QUANTIFICATION SOFTWARE#
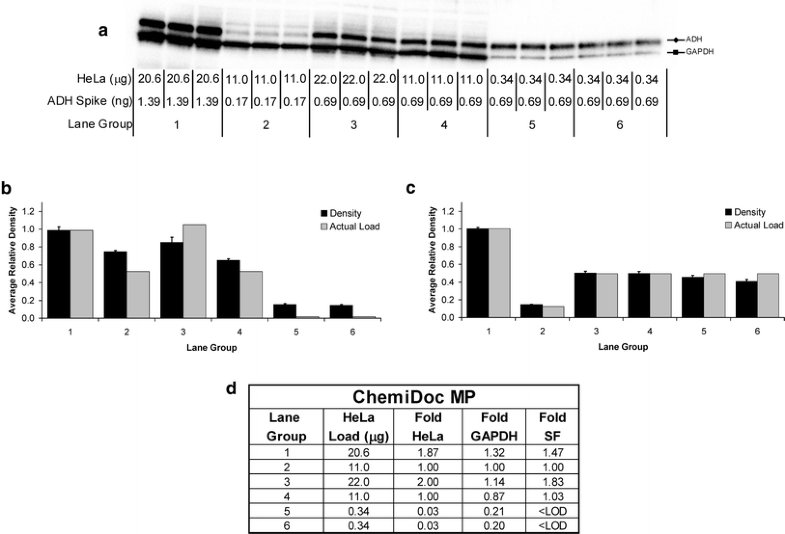
The software interprets the raw data in three dimensions with the length and width of the band defined by the “Lanes and Bands” tool in concert with the “Lane Profile” tool such that the chemiluminescent signal emitted from the blot is registered in the third dimension as a peak rising out of the blot surface. ImageLab software version 4.1 (Bio-Rad) was used for image acquisition and densitometric analysis of the gels, blots, and film in this study. Image acquisition and densitometric analysis. Based on our findings, we propose a rigorous and simple methodology to produce high quality, reproducible, and quantitative western blot data. Here, we will demonstrate how standardized protein samples, when processed with film versus digital imaging methods and different normalization approaches, produce vastly different results. These new tools and techniques eliminate the limitations associated with film-based detection and meet the journal reviewers’ demands for quantifiable protein expression data. By contrast, the rapid evolution of affordable and highly sensitive gel and blot imaging technologies coupled with new reagents gives researchers the means to produce truly quantitative western blot data-as long as the process is carried out with proper technique, validation, and controls. However, unless the experiments are performed with a deep understanding of these limitations, this method of detection is an approximation at the best and often nonquantitative if used inappropriately. The scientific community has largely ignored these challenges, mostly because of a common misperception that film produces the highest quality data from western blots. These problems stem from a low-dynamic range of detection and the difficulty in accurately determining the limit of detection. Thus, editors and reviewers of scientific journals are looking at western blot results, particularly at the densitometric analysis to determine the fold differences in protein expression, with greater scepticism, often requesting the raw data files.Ĭhemiluminescent western blot data, derived from film-based detection, poses distinct challenges in producing quantifiable, reproducible data. This underlines the negative perception by which the scientific community views the western blot data. A recent report predicts that approximately 25 % of the accepted papers include at least one inappropriately manipulated figure and many of these are associated with western blotting. These include challenges related to every step of the western blotting procedure, from sample preparation, normalization, SDS–PAGE gel loading, protein transfer, primary and secondary antibody selection, incubations, and washes, detection method selection to densitometric analysis. However, there are many potential stumbling blocks in this procedure that can preclude reliable results. This multistep method determines the presence or absence, size, and modification or degradation states of target proteins, as well enables the quantitation of proteins from complex protein mixtures or homogenates. Western blotting has been a staple in life science labs for several decades-ever since researchers published the first detailed description of this protein detection technique in 1979.


 0 kommentar(er)
0 kommentar(er)
